At the time of writing this article, 2,898 clinical studies related to COVID-19 are registered on WHO’s International Clinical Trials Registry Platform. This portal, established in 2004, stores the information about trials from all around the world and makes them available to everyone online from a single point of access. To be registered, a trial must provide a set of internationally-agreed-upon information about its design, conduct, and administration.
Why do we need this registry? Well, WHO believes that the registration of clinical trials is a “scientific, ethical and moral responsibility.” It also avoids duplication and recruits more participants. Either way, trial transparency is a big deal, and it’s especially important in times when global collaboration and connectivity is crucial for our wellbeing.
Today we will talk about how clinical trials are done today from the technology point of view. Spoiler alert: There are a few areas ripe for improvement. We’ll disclose how these issues can be resolved.
What is a clinical trial? Clinical trial workflow and toolkit
A clinical trial is an experiment done to ensure the safety and efficacy of new treatments. A pharmaceutical, biotechnical, or medical device company sponsors the clinical trial to get their medication or device approved by FDA or an international regulatory authority. They often employ a contract research organization (CRO) to plan and conduct the research on behalf of the sponsor. It’s usually done in four stages. It starts with lab testing, followed by human testing. At every subsequent stage, dosage is adjusted and the number of participants is increased.
There are four main types of objectives in clinical trials:
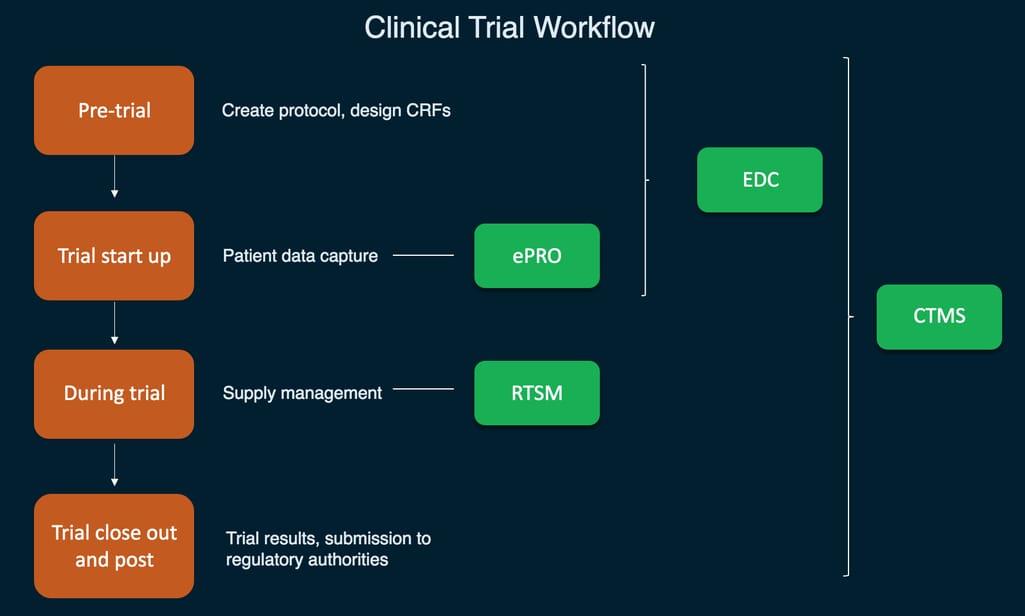
- Study design and patient data capture
- Patient outcome collection
- Supply management
- Centralized trial management
A simplified visualization of main clinical trial systems and their functions
Study design and patient data capture using EDC
There are two main documents compiled during trial design - protocols and case report forms (CRFs).
A protocol describes the objective, methodology, evaluation criteria, and even trial location. It’s compiled by a group of experts, and in the US, EU, and Japan, a clinical trial protocol should adhere to the Good Clinical Practice guidance -- an international ethical and scientific standard for the design and conduct of clinical trials.
A case report form is a questionnaire that documents the data about each participant and can include as little as information about their current physical condition and as much as data spanning several weeks or months. Today, a lot of case report forms are electronic (eCRFs) and they are created and filled using Electronic Data Capture (EDC) software.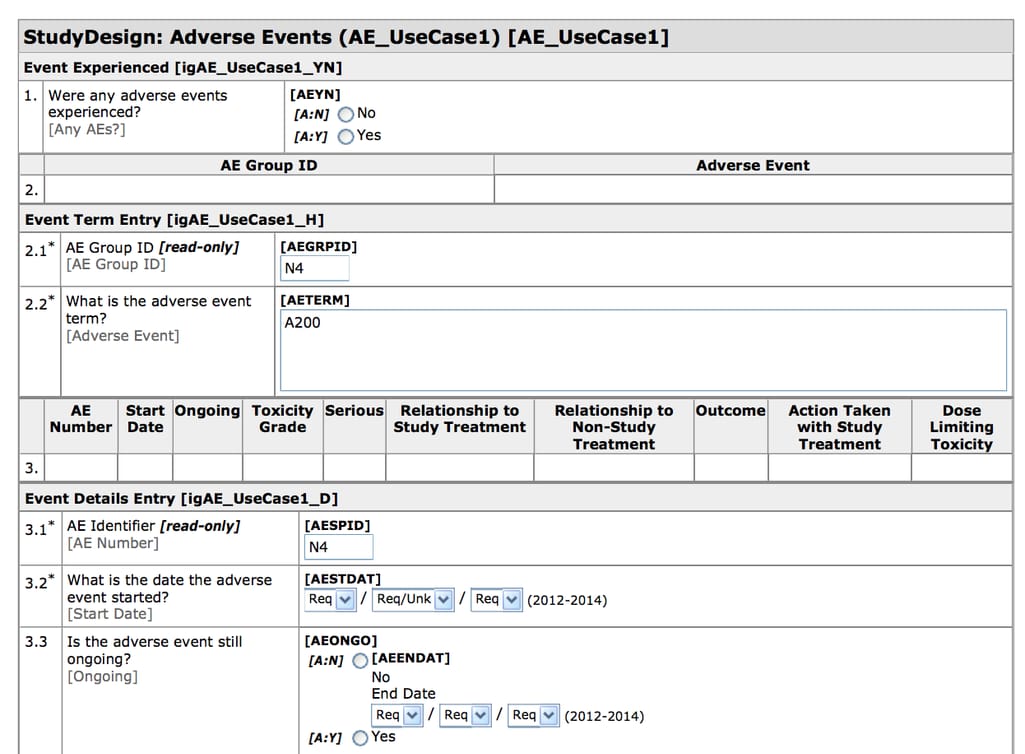
CDASH-compliant eCRF created in Oracle Health Sciences InForm Source: CDISC
Electronic Data Capture is a tool for recording and collecting clinical documents. EDCs have CDISC-compliant templates making it easy to design and export eCRFs. EDC systems are the most widely used eClinical tools with the top solutions including Oracle Health Sciences InForm, Medidata Rave EDC, Bioclinica EDC, and many more.
EDCs can be as complex or as simple as the study team dictates, but in its core, EDC consists of a graphical user interface, a query management module, and a reporting tool. Today, EDC has replaced Clinical Data Management Systems (CDMS) - another acronym that refers to the process rather than to any specific tool. Clinical Data Management is the practice of delivering the best data quality with the least number of mistakes. So basically, EDC is a CDMS, and the terms may be used interchangeably
Today, clinical studies mostly use EDCs to collect CRFs, but modern solutions can go way beyond that. With the right integrations, EDCs can receive data from apps and medical devices, Electronic Health Records (EHRs) at the hospitals, and ePRO questionnaires.
Patient outcome collection using ePRO
We’ve already established eCRFs as the main source of data in clinical trials making up 78 percent of data managed in EDC. The rest are lab results and data from eCOA (clinical outcome assessment) and more specifically - ePROs.
ePROs or electronic Patient-Reported Outcomes are patient-facing tools allowing study participants to monitor and report their health. They often look like questionnaires and can be accessed via a pre-installed app on a tablet or smartphone, usually provided by the trial sponsor. ePROs are often custom-made with the largest vendors including ERT, CRF Health, Parexel, Medidata, and more.
ePRO data should be compliant with regulatory applications, because otherwise you wouldn’t be able to use it for FDA approval. So, most ePRO surveys are completed on site. That means that the biggest challenge is also the biggest opportunity - creating a compliant ePRO solution that can be used on a patient’s own device will speed up integration and reduce trial costs.
Supply management using IRT and RTSM
An actual trial is often done at hospitals with nurses and physicians acting as coordinators and investigators, who use different systems to manage the trial and avoid bias. Two main functions of clinical trials - randomizing subjects and managing drug supply - are traditionally managed by Interactive Response Technology (IRT). You can also meet acronyms like Interactive Voice Response Systems (IVRS), Interactive Web Response Systems (IWRS), and RTSM - Randomization and Trial Supply Management.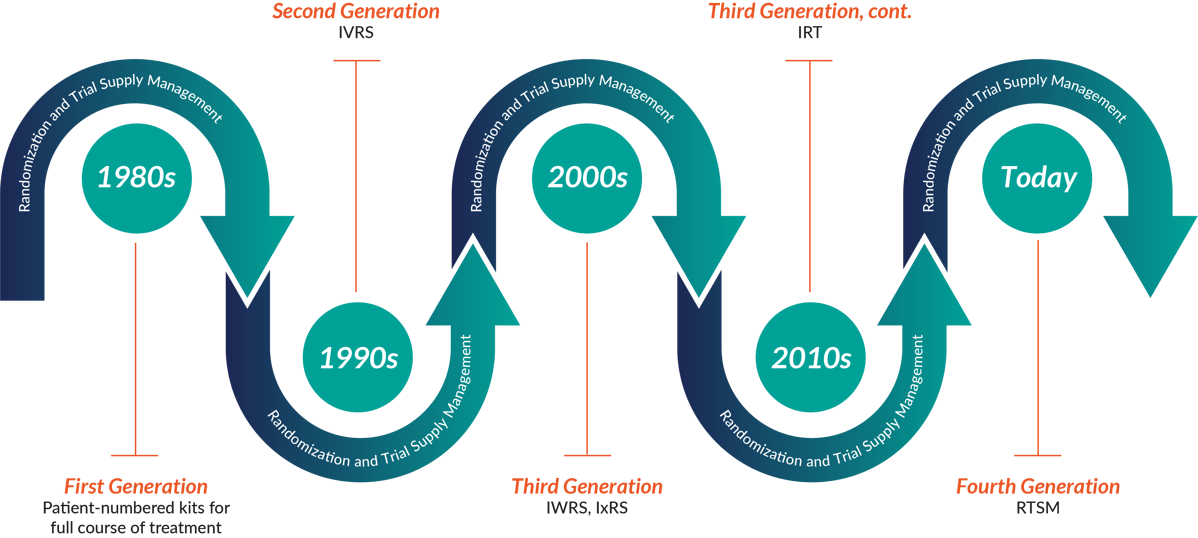
RTSM Through The Ages - 1980s to Now Source: 4Gclinical
All of them practically mean the same type of software - the systems used to randomize study participants (the ones receiving placebo vs the real drug) and dispensing medications to them. Tools like this use adjustable algorithms, sometimes even using Natural Language Processing and Machine Learning to understand your trial requirements and prevent drug waste. The naming will solely depend on what the vendor decides to call it, for example Medidata Rave RTSM, Medpace ClinTrak IRT, or ITclinical IRT:IWRS.
Centralized trial management using CTMS
Clinical Trial Management System (CTMS) is a software used to manage and streamline the whole clinical research workflow. It’s usually used by a research sponsor (pharmaceutical, biotechnical, or medical device company) or a Contract Research Organization (CRO), employed by a sponsor to operate the trial on their behalf.
So, in short, CTMS is a project management tool allowing you to track the progress and set milestones, see how each team is doing, schedule subject visits, manage relationships between different members of the team, control costs, and of course, prepare reports.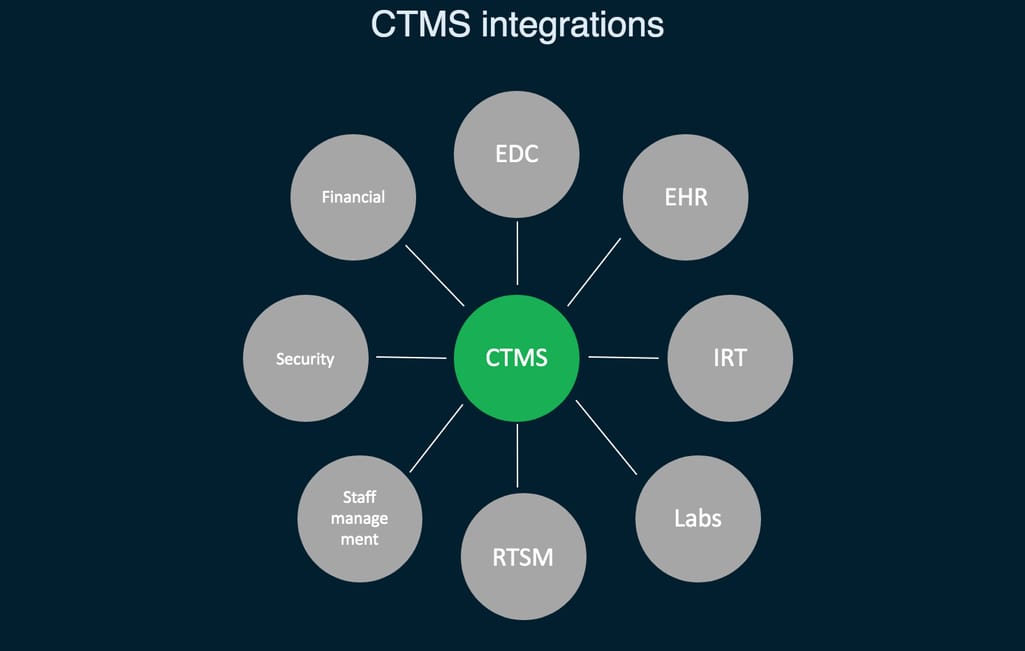
CTMS integrations needed for the centralized trial management
It seems as though CTMS may solve the integration and uniformity problem as many modern solutions come with EDC support. Along with previously mentioned Oracle, Bioclinica, and Medidata, popular systems include Parexel MyTrials, Forte OnCore, and more. But many of such systems already used in the field are outdated.
Clinical trial challenges
Did you notice that there are a few common issues present at every stage of conducting a trial? We can sum them up into three big challenges.
Long and complex R&D processes. The drug R&D process may take years and it’s getting more expensive and demanding with each passing decade. The volume of produced data is increasing faster than our understanding of how to manage it effectively. How do we improve it? Certainly not with introducing more software. Which leads us to the next challenge.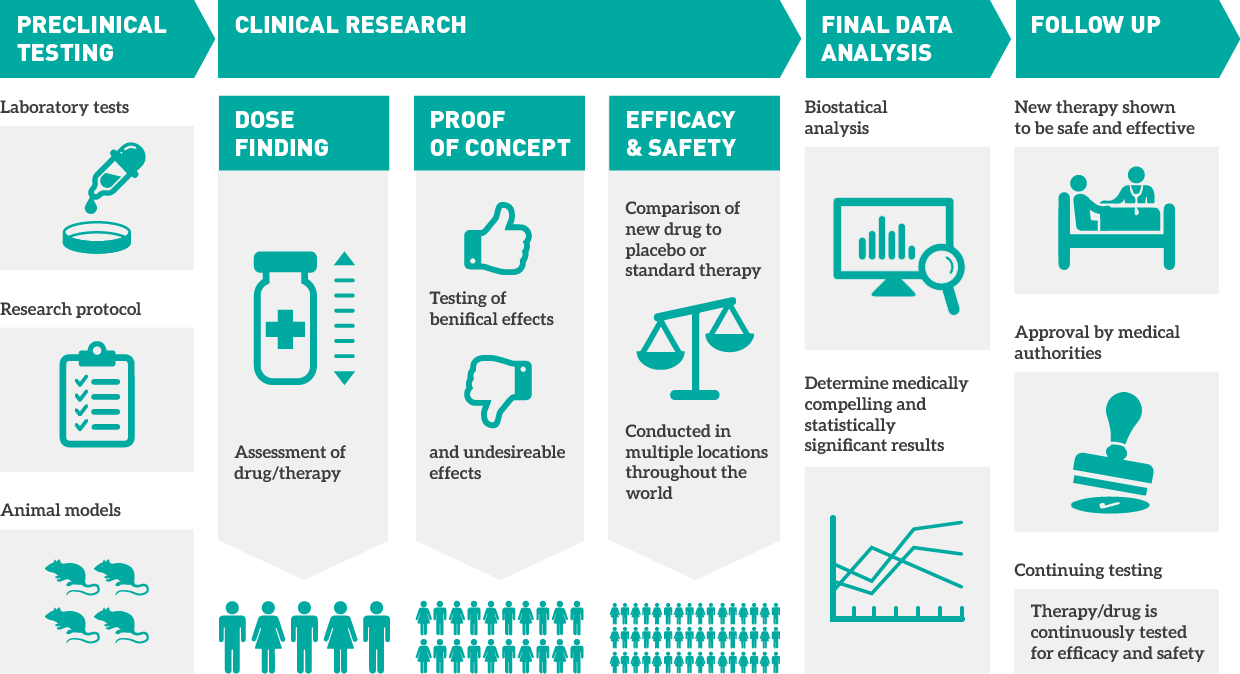
Bringing a drug to market takes from months to years Source: ProQR
No uniformity. Today, studies have to balance up to ten different systems at the same time, with an average of four data sources used in clinical studies. Data comes from everywhere, from ePRO questionnaires to dedicated apps, wearables, electronic informed consent forms, and so on. Should we mention that all this variety makes it impossible to quickly and effectively consolidate and use all that data for analysis? There have been, however, efforts to bring consistency to clinical study documents and standardize data exchange. We will cover these new possibilities further.
Difficulty of integration. We introduced technology to get rid of error-prone paper documents, and stop duplicating sensitive data, but more problems surfaced. Study teams adopt technology at different paces and the variety of tools makes it impossible to completely automate the process. Data is often sent via email or manually copied, but new systems are constantly being adopted. We come full circle to the first challenge - too much data and no idea how to put it to good use.
How to solve clinical trial challenges
We have to be realistic about any change, especially when it occurs in such a heavily regulated domain as clinical research. Yes, we can achieve uniformity by scrapping the whole passel of tools and replacing them with a single, integrated system, but we can’t expect from study teams and sponsors to embrace this change. Since we have to work with what we have, here are some available solutions.
Adopting clinical data standards by CDISC
CDISC or Clinical Data Interchange Standards Consortium is a standards developing organization that supports data exchange in clinical research. Their standards are universal, free to use, and easily customizable to all types of research.
CDISC offers both data content standards that provide a blueprint for data collection and data exchange standards that help exchange data across the whole healthcare ecosystem, using an XML protocol.
Basically, CDISC allows for bringing the lacking uniformity to clinical documents and integrations between different systems.
Using CDISC standards is not obligatory, but very much encouraged by regulatory authorities like FDA - the submission and review process is usually faster because all your documents are submission-ready at the design stage already. We have a whole article explaining different CDISC standards and how to approach their adoption, so be sure to check it out.
Automating EHR-EDC connectivity with the OneSource model
There’s a huge gap between how data is managed in healthcare and clinical trials. This wasn’t a problem before when all data was captured on paper. But now, when almost all of it is recorded digitally, in EHRs and EDC, it can’t be easily accessed by both parties. This is especially ineffective considering that EDC data is initially recorded to be later used in healthcare, and EHRs keep data records to be later used in research, but the connectivity is just not there.
So, FDA along with the University of California San Francisco (UCSF) and Stanford Centers of Excellence in Regulatory Science and Innovation (CERSI) developed a method to automate the flow of data from EHRs to EDC. It’s called OneSource. OneSource serves as the “single source of truth” where all data from ePROs, EHRs, and EDC is compiled, mapped, and displayed according to clinical data standards such as CDISC, HL7, and FHIR.
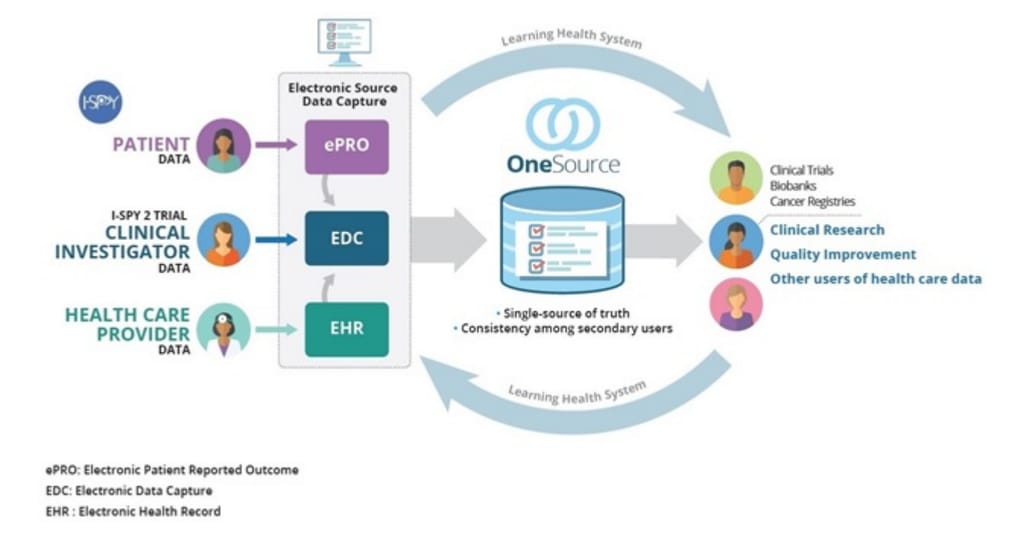 Source Data Capture from EHRs: Using Standardized Clinical Research Data Source: FDA
Source Data Capture from EHRs: Using Standardized Clinical Research Data Source: FDA
OneSource development framework with an example of implementation using OpenClinica EDC is publicly available. It’s stated that any organization with access to technical tools and domain-skilled team can provide the same level of data harmonization.
Modernizing legacy CTMS software
Clinical Trial Management Systems were first introduced 20 years ago. This cumbersome on-premise application can no longer support modern clinical research processes and satisfy the demands of sponsors and CROs. There are two ways you can deal with them:
- Migrate to a new CTMS and change your entire workflow
- Modernize an old CTMS and keep your workflow intact
We recommend the second approach. First, because CTMS exists to support people and having a study team entirely change how they do their job is difficult and ineffective. Second, because it allows you to make custom changes, often unavailable in out-of-the-box solutions, and do them painfully, without unplugging the whole enterprise.
Either way, the industry needs to face its struggles and start dealing with them one by one. If it doesn’t, the potential for drastic improvements will remain lost.

Maryna is a passionate writer with a talent for simplifying complex topics for readers of all backgrounds. With 7 years of experience writing about travel technology, she is well-versed in the field. Outside of her professional writing, she enjoys reading, video games, and fashion.
Want to write an article for our blog? Read our requirements and guidelines to become a contributor.